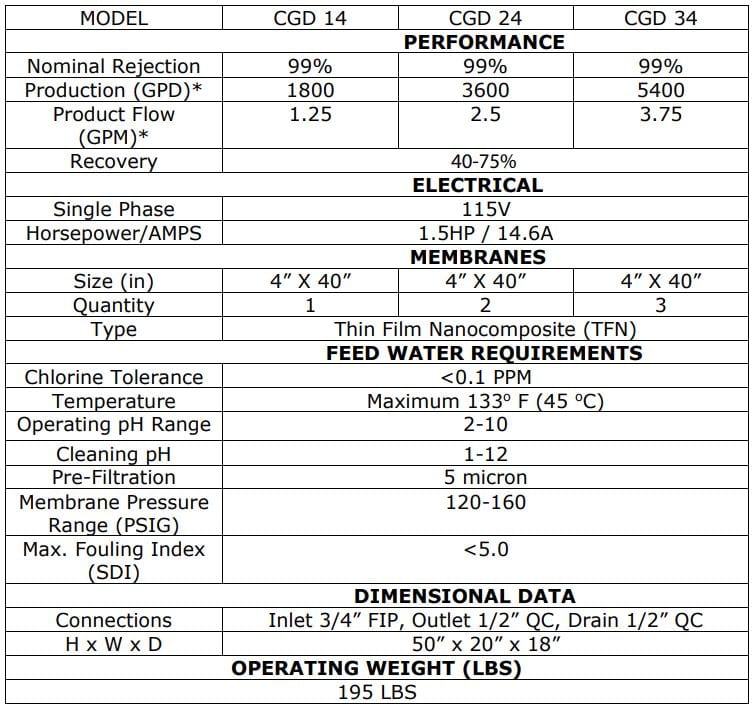
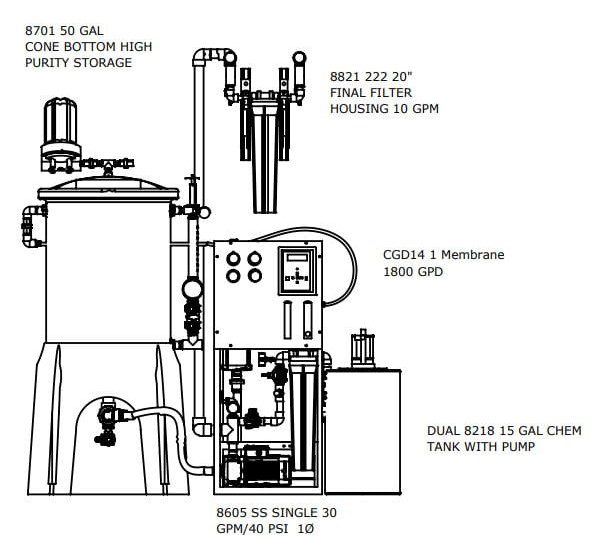
The Critical Water Systems are designed to meet or exceed the proposed standards
The ANSI/AAMI ST108 standard, also known as the “Water for the processing of medical devices” standard, is a comprehensive set of guidelines for the design, construction, and operation of Critical Water Departments . The standard outlines the minimum requirements for water quality and steam purity necessary to effectively process medical devices intended for use on a patient. This standard was developed by the Association for the Advancement of Medical Instrumentation (AAMI) and is recognized as the gold standard for operations.
ChemREADY offers comprehensive solutions for ANSI/AAMI ST108 standard critical water systems, designed to meet stringent sterilization and disinfection standards. Our products are ideal for ensuring optimal performance and compliance, making them essential for Sterile Processing Departments and Ambulatory Surgical Centers. With a focus on quality and efficiency, our ST108 equipment helps maintain the highest standards of cleanliness and safety.
Our ST108 standard critical water systems play a crucial role in Sterile Processing Departments, ensuring the consistent supply of high-purity water necessary for sterilization processes. Additionally, these systems support the rigorous demands of Ambulatory Surgical Centers, providing the sterile environment crucial for patient safety. By integrating our advanced equipment in these settings, facilities can ensure adherence to ANSI/AAMI standards, enhancing overall operational efficiency.
AAMI ST108 will not allow for direct feed systems in critical water processing any longer, and thank goodness! The loop velocity of 3-5 feet per second being recirculated continuously through a 0.2 final filter will ensure that the last water that touches surgical instruments is free of bacteria and endotoxins. This means less surgical site infections!!
The technical information report 34 (TIR34) converted to Standard ST108 and covers the selection and maintenance of effective water quality suitable for reprocessing medical devices in critical water water facilities. It provides guidelines for selecting the water quality necessary for the reprocessing of categories of medical devices and addresses water treatment equipment, water distribution and storage, quality control procedures for monitoring water quality, strategies for bacterial control, and environmental and personnel considerations.
Water can be treated by a variety of methods that yield different levels of water quality. In general, as the chemical quality of water improves, its microbial content could increase unless the system is closely monitored to prevent microbial overgrowth. Gram-negative bacteria and nontuberculous mycobacteria can grow in any type of water, including tap, softened, deionized (DI), reverse osmosis (RO) treated, and distilled water. The rate of growth and the microbial levels attained are a function of the amount of organic contaminants in the water. The importance of monitoring water quality to prevent problems with microbial overgrowth cannot be overemphasized.
The AAMI ST108 Critical Water Water Systems are designed to meet or exceed the proposed standards put forth. The improved process water used in Water Systems lowers infection, protects equipment and improves patient outcomes.
Our ANSI/AAMI ST108 standard critical water systems include advanced features such as automated monitoring, reliable performance, and ease of use, making them ideal for healthcare settings. By integrating these solutions, Sterile Processing Departments and Ambulatory Surgical Centers can achieve consistent and effective sterilization processes, maintaining the highest levels of hygiene and safety.
The best practice design provides for larger flow RO with a smaller fresh storage for your Critical Water Delivery System. The design is compliant with AAMI TIR34 and brass/copper/lead free with rust free powder coated aluminum frame. Our deluxe controller with conductivity and optional facilities management communications package make it the most advanced solution on the market.